
This shortcode is only available on vendor store pages.

الحصن الحصين البيطرية

This shortcode is only available on vendor store pages.
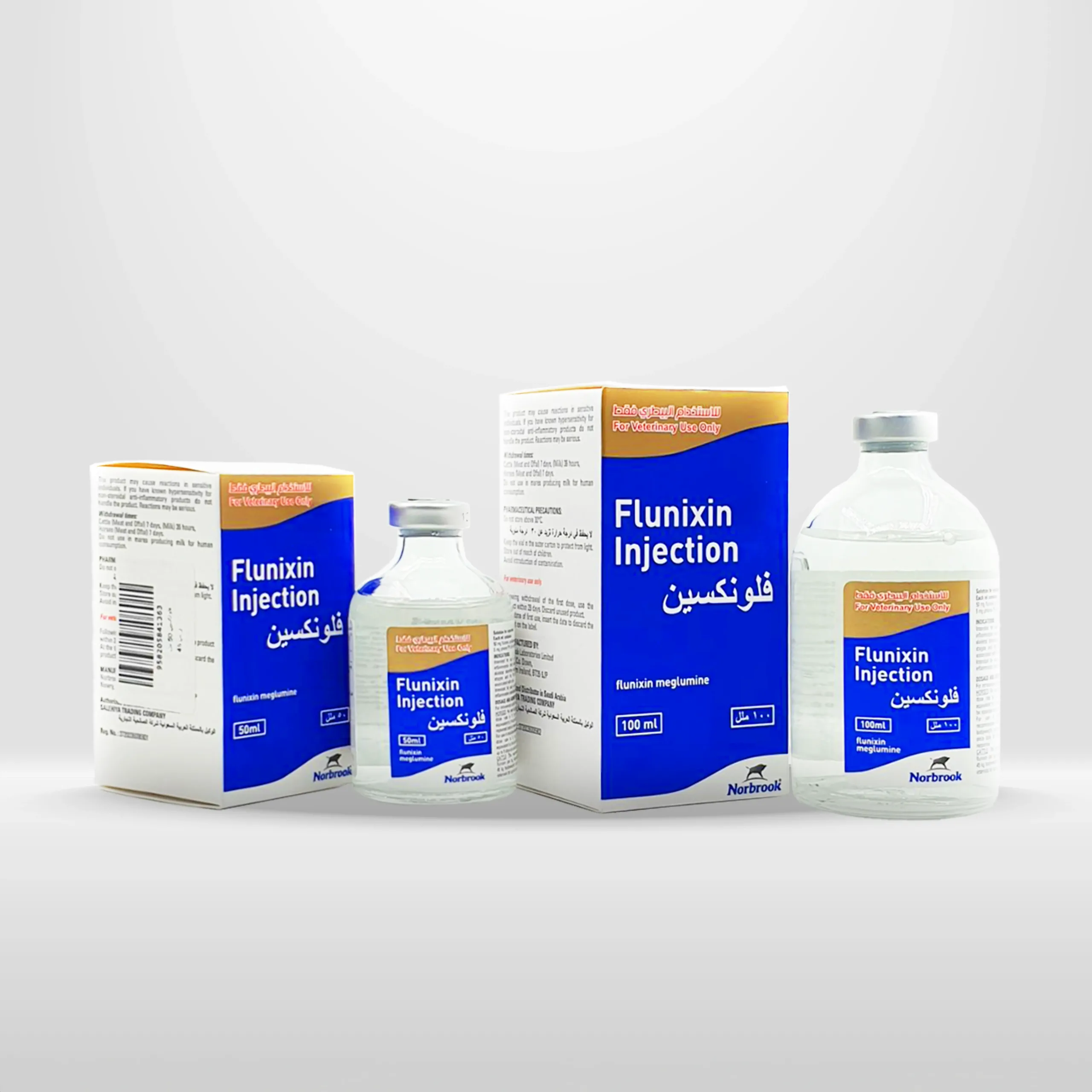
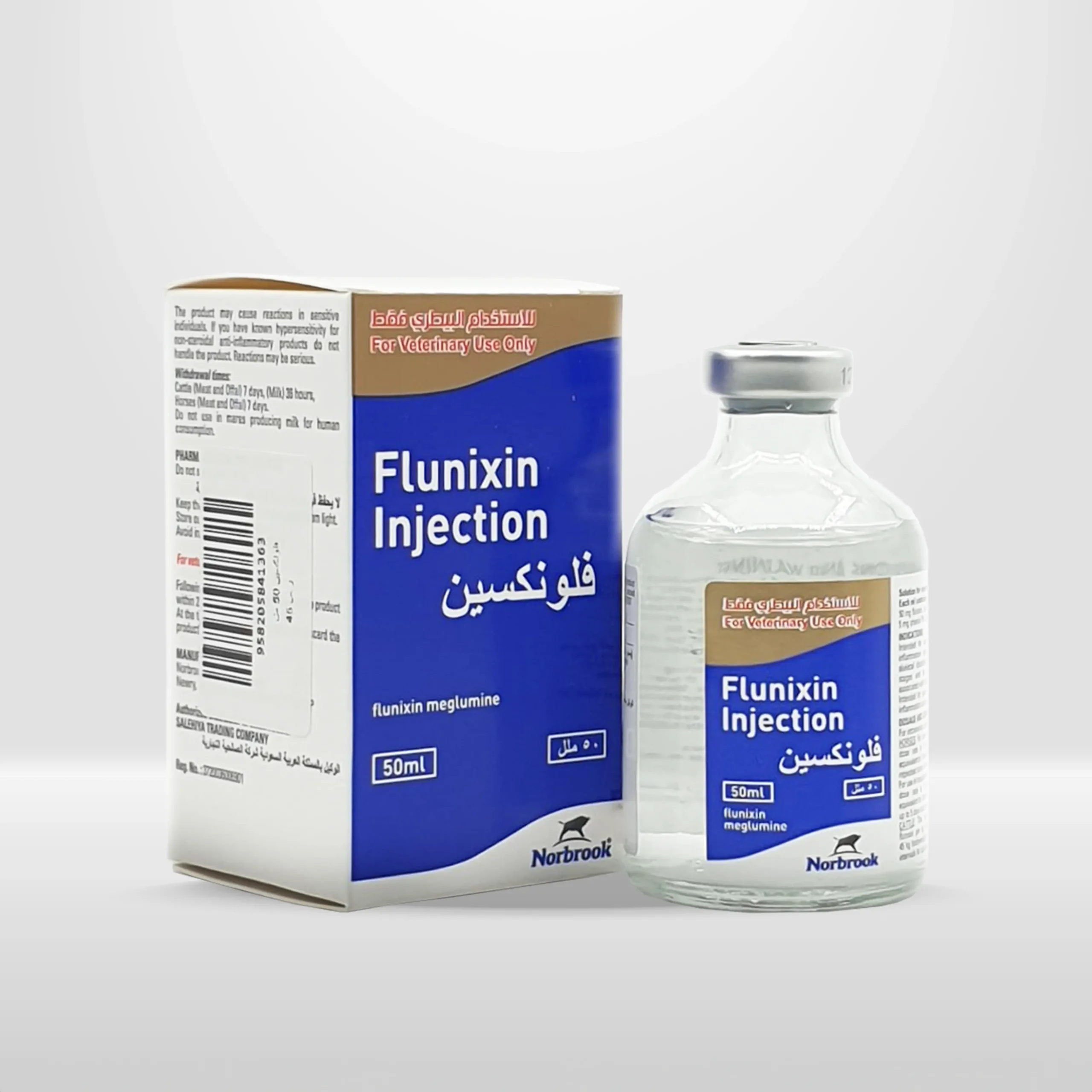
Flunixin | anti-inflammatory for Cattle and Horses
اشتري من صيدليات أخرى
For Veterinary Use Only
In the horse, Flunixin Injection is indicated for alleviation of inflammation and pain associated with musculoskeletal disorders alleviation of visceral pain associated with colic. In cattle, Flunixin Injection is indicated for control of acute inflammation associated with respiratory disease.
Ingredient
Each ml Flunixin contains
Flunixin, as flunixin meglumine,
Phenol, as preservative
Sodium Formaldehyde
Sulfoxylate Dihydrate
A clear colorless solution.
Indications
In the horse, Flunixin Injection is indicated for the alleviation of inflammation and pain associated with musculoskeletal disorders and for the alleviation of visceral pain associated with colic.
In cattle, it is indicated for the control of acute inflammation associated with respiratory disease.
it may also be used as adjunctive therapy in the treatment of acute mastitis.
Contraindications:
Do not exceed the recommended dose of the product or the duration of treatment.
Use is contraindicated in animals suffering from cardiac, hepatic or renal disease, where there is the possibility of gastrointestinal ulceration or bleeding where there is evidence of a blood dyscrasia or hypersensitivity to the product.
Do not use it in dehydrated animals suffering from ileus-associated colics.
Do not use it within 48 hours before expected parturition in cows.
Target species:
Cattle and Horses
Dosage,route of administration:
Flunixin Injection is indicated for intravenous administration to cattle and horses.
Do not exceed the recommended dose or duration of treatment.
HORSES:
For use in equine colic, the recommended dose rate is 1.1 mg flunixin/kg bodyweight equivalent to 1 ml per 45 kg body weight by intravenous injection.
Treatment may be repeated once or twice if colic recurs.
For use in musculoskeletal disorders, the recommended dose rate is 1.1 mg/kg bodyweight equivalent to 1 ml per 45 kg BW injected intravenously once daily for up to 5 days according to clinical response.
For the treatment of endotoxemia or septic shock associated with gastric torsion and other conditions in which the circulation of blood to the gastrointestinal tract is compromised: 0.25mg/kg .(1 ml per 200 kg) every 6-8 hours
CATTLE:
The recommended dose rate is 22 m/kg bodyweight equivalent to 2 ml per 45 k injected intravenously and .repeated .24 hour interval up to 5
consecutive days
Withdrawal period:
Animals must not be slaughtered for human consumption during treatment.
Cattle and Horses may be slaughtered for human consumption only after 7 days from the last treatment.
Milk for human consumption must not be taken during treatment
Milk for human consumption may only be taken from treated cows after 36 hours from the last treatment
Do not use the product in mares producing milk for human consumption.
Special storage precautions:
Animals must not be slaughtered for human consumption during treatment.
Cattle and Horses may be slaughtered for human consumption only after 7 days from the last treatment.
Milk for human consumption must not be taken during treatment
Milk for human consumption may only be taken from treated cows after 36 hours from the last treatment
Do not use the product in mares producing milk for human consumption.
Overdose:
Overdose studies in the target species have shown Flunixin to be well tolerated. Overdosage is associated with gastrointestinal toxicity.
Packaging quantities:
Multi-dose vials of 50 ml, 100 ml, and 250 ml
It is also presented in packs of 5, 10, and 12 vials for the 50 ml and 100 ml and a pack of 5 vials for the 250 m.
| Size | 100 ML, 50 ML |
|---|
Vendor Information
- Store Name: الحصن الحصين البيطرية
- Vendor: الحصن الحصين البيطرية
- Address: Riyadh
- No ratings found yet!
Out of stock
Curasol C30 | Betaine helps in coccidiosis treatment in animals.
Sold by الحصن الحصين البيطريةFurex750 | furosemide | diuretics | for animals
Sold by الحصن الحصين البيطريةOut of stock
Out of stock
Selozn E 20% | Zn, vitamin e and selenium for poultry & animals
Sold by الحصن الحصين البيطريةAVIMEC | Ivermectin | antiparasitic drug | for animals
Sold by الحصن الحصين البيطريةProduct Enquiry
Related Products
-
Flunixin | anti-inflammatory for Cattle and Horses
Sold by صيدلية البعادي البيطريةRated 0 out of 545 ر.س – 75 ر.سPrice range: 45 ر.س through 75 ر.س -
Out of stock
-
Out of stock












Reviews
There are no reviews yet.